Therapies engineered to prolong clotting factor protein circulation time manipulate the balance of pro-coagulant and anti-coagulant proteins or introduce new genetic material to enable endogenous factor protein production dominate the clinical trial landscape of hemophilia. Hemophilia B or Christmas disease is a genetic bleeding disorder resulting in the lack of ability to produce blood-clotting factor IX FIX.
 New Gene Therapy For Hemophilia Elearning Module Hemophilia World News
New Gene Therapy For Hemophilia Elearning Module Hemophilia World News
Find a Pfizer study thats right for you by searching for conditions keywords or a National Clinical Trials NCT number.

Hemophilia clinical trials. Seasonal allergies at time of dosing or clinical findings at Screening. A randomized clinical trial of prophylaxis in children with hemophilia A the ESPRIT Study This randomized trial confirms the efficacy of prophylaxis in preventing bleeds and arthropathy in children with hemophilia particularly when it is initiated early in life. 1Hematology and Transfusion Medicine Paul-Ehrlich-Institut Langen Germany.
Viiala NO1 Larsen SR Rasko JE. - Male 18 years of age. Individuals with hemophilia B suffer repeated bleeding events which can cause chronic joint disease and sometimes leads to.
Clinical trials and technical tribulations. A Trial Comparing Nonacog Beta Pegol N9-GP and ALPROLIX in Patients With Haemophilia B Rochester MN This trial is conducted in Europe and the United States of America. As monogenic disorders hemophilia A and B are compelling candidates for treatment with gene therapy.
In this study we explored the potential effect of emicizumab prophylaxis on joint health scores and biomarkers of bone health in PwHA without factor VIII FVIII inhibitors who participated in the HAVEN 3 clinical trial. Clinical trials and registries in haemophilia. This adaptive design is necessary as randomized trials in rare diseases are otherwise not possible.
Subjects anticipating elective surgery or other invasive procedure within 1. C3731003 is a pivotal Phase 3 study to evaluate the clinical efficacy and safety of a single IV infusion of PF-07055480 Recombinant AAV26 Human Factor VIII Gene Therapy in adult male participants with moderately severe or severe hemophilia A FVIIIC1 for the study duration of 5 years. The purpose of this study is to determine the frequency of bleeding episodes in patients receiving fitusiran as prophylactic treatment of hemophilia compared with patients who are assigned to continue with their regular medication.
The INHIBIT Clinical Trials Platform includes two linked trials the Inhibitor Prevention Trial Prevention Trial and the Inhibitor Eradication Trial Eradication Trial that will be conducted at up to 41 US. HA is a serious blood coagulation disorder caused by a deficiency in FVIII that results in a failure to produce FVIII in sufficient quantities to achieve satisfactory haemostasis. Comparison of PUP data derived from different data sources.
Milwaukee June 16 2020 Patients are now able to participate in a phase I first-in-human FDA approved clinical trial seeking a potential long-term treatment for Severe Hemophilia A using a gene therapy that targets synthesis of coagulation Factor Eight FVIII which is stored and released from blood platelets at the site of an injured blood vessel. The Hemophilia Joint Health Score HJHS is a tool used to assess joint function and gait with a lower score indicating better joint health. In addition the study will assess safety quality of life pharmacodynamics PD and pharmacokinetics PK.
The introduction of recombinant factor concentrates in the early 1990s facilitated the use of prophylactic replacement as standard care for hemophilia rather than on-demand treatment. 1Gene and Stem Cell Therapy Program Centenary Institute University of Sydney NSW Australia. The current single country multi-centric open label non-randomized pragmatic clinical trial is a post-approval study to fulfill the Central Drugs Standard Control Organization CDSCO request for supplementary information relating to the use of moroctocog.
This study will capture different approaches in the management of persons with haemophilia A HA and inhibitors. Gene therapy for hemophilia. Documented Human Immunodeficiency Virus HIV.
This is a multi-center randomized Phase III Clinical Trials Platform INHIBIT in which hemostatic agents will be compared using adaptive design to prevent and eradicate inhibitors in patients with severe hemophilia A. The aim of this trial is to compare the pharmacokinetics the exposure of the trial drug in the body of nonacog beta pegol N9-GP and ALPROLIX in patients with haemophilia B. Diagnosed with any other bleeding disorder in addition to hemophilia A.
The factors or reasons that allow a person to participate in a clinical study. Keipert C1 Jonker CJ2 van den Berg HM3 Hilger A1. A surge in therapeutic clinical trials over recent years is paving the way for transformative treatment options for patients with hemophilia.
- Severe hemophilia A past evidence of circulating FVIII activity of 1 normal - Treated or exposed to FVIII concentrates or cryoprecipitate for at. Hemophilia treatment centers HTCs affiliated with universities. The clinical hold was initiated following the submission of a safety report in mid-December relating to a possibly related serious adverse event associated with a preliminary diagnosis of hepatocellular carcinoma HCC a form of liver cancer in one patient in the HOPE-B trial that was treated with etranacogene dezaparvovec AMT-061 in October 2019.
You can also see nearby clinical trials.
 New Gene Therapy For Hemophilia Elearning Module Hemophilia World News
New Gene Therapy For Hemophilia Elearning Module Hemophilia World News
 Clinical Trials For Gene Therapy Of Hemophilia Download Table
Clinical Trials For Gene Therapy Of Hemophilia Download Table
Bioverativ Taps Patient Social Network For Input On Hemophilia Trial Design Fiercepharma
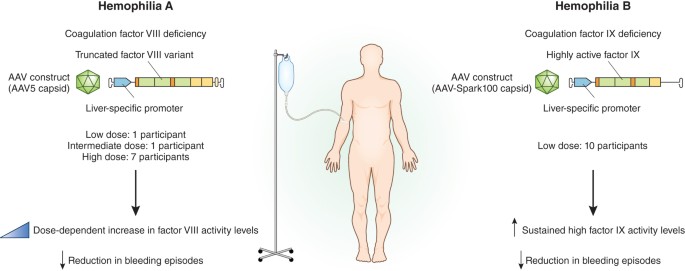 Gene Therapies For Hemophilia Hit The Mark In Clinical Trials Nature Medicine
Gene Therapies For Hemophilia Hit The Mark In Clinical Trials Nature Medicine
 Preclinical Studies And Clinical Trials On Gene And Cell Therapy For Download Table
Preclinical Studies And Clinical Trials On Gene And Cell Therapy For Download Table
 New Gene Therapy Cme Activity Updates On Clinical Trials Of Gene Therapy In Hemophilia Hemophilia World News
New Gene Therapy Cme Activity Updates On Clinical Trials Of Gene Therapy In Hemophilia Hemophilia World News
 Fda Guidance For Human Gene Therapy For Hemophilia A B
Fda Guidance For Human Gene Therapy For Hemophilia A B
 Randomized Clinical Trials Concerning Rfviia In Hemophilia Patients Download Table
Randomized Clinical Trials Concerning Rfviia In Hemophilia Patients Download Table
 Recently Approved Or In Clinical Trial Novel Hemophilia A Therapeutics Download Table
Recently Approved Or In Clinical Trial Novel Hemophilia A Therapeutics Download Table
 Hemophilia Clinical Trials Free Whitepaper
Hemophilia Clinical Trials Free Whitepaper
 A Molecular Revolution In The Treatment Of Hemophilia Sciencedirect
A Molecular Revolution In The Treatment Of Hemophilia Sciencedirect
 Update On Efficacy Of Clinical Trials
Update On Efficacy Of Clinical Trials
Hemophilia Therapy The Future Has Begun Haematologica
 Clinical Trials For Gene Therapy Of Hemophilia Download Table
Clinical Trials For Gene Therapy Of Hemophilia Download Table
Comments
Post a Comment