BrainStorm Cell Therapeutics Inc. Tirasemtiv is a drug that affects skeletal muscle receptors.
 Brainstorm Cell Therapeutics Passes Safety Milestone In Phase 3 Trial Of Nurown Therapy For Als Biobanking Com
Brainstorm Cell Therapeutics Passes Safety Milestone In Phase 3 Trial Of Nurown Therapy For Als Biobanking Com
The company has no plans to continue ALS work said CEO Damian Marron but hopes olesoxime will be effective for.

Als phase 3 trials. BrainStorm Cell Therapeutics announced that the Phase 3 clinical trial of its cell therapy candidate NurOwn in 200 amyotrophic lateral sclerosis ALS patients is fully enrolled and treatment is. The study will enroll a broad ALS population from North. To test the effectiveness of the two-drug combination the researchers recruited 137 ALS.
The trial NCT04768972 will enroll up to 64 people ranging in age from 12 to 65 who are not on permanent ventilation. And Europe the result of a collaboration between. The ALS Association plans on putting out a public petition to help fast-track expanded access of the drug or even approval without the standard.
But Trophos announced on their website on 13 December that the drug failed to achieve the desired survival effect in a Phase 3 clinical trial. Enrollment is currently ongoing in South Korea and patients interested in participating must be able to stay in the country for at. A pivotal trial testing spinal injections of NurOwn against placebo in safely treating ALS enrolled 200 patients with results likely in October 2020.
The announcement was made in a. The Phase 2b3 clinical trial is a multi-center two-arm randomized double blind placebo controlled trial that will compare MN-166 to placebo in approximately 230. Amyotroph Lateral Scler Frontotemporal Degener.
The trial is now enrolling and aims to enroll approximately 60 people with SOD1 ALS. Biogen has initiated a phase 3 clinical trial evaluating tofersen previously called BIIB067 an antisense oligonucleotide ASO a type of antisense drug targeting superoxide dismutase SOD1 for the potential treatment of ALS. BrainStorm has fully enrolled a Phase 3 pivotal trial in ALS NCT03280056 investigating repeat-administration of autologous MSC-NTF.
If a phase 2 study generates positive results the FDA typically requires a larger and longer phase 3 trial. A global Phase 3 trial of AMX0035 as an oral treatment for amyotrophic lateral sclerosis ALS intends to enroll up to 600 patients the therapys developer Amylyx Pharmaceuticals announced in a press release. At this weeks 2019 American Academy of Neurology AAN Annual Meeting in Philadelphia Biogen.
Phase 3 Study of Dexpramipexole in ALS - Full Text View - ClinicalTrialsgov Phase 3 Study of Dexpramipexole in ALS EMPOWER The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Phase III studies are randomized controlled multicenter trials on large patient groups 3003000 or more depending upon the diseasemedical condition studied and are aimed at being the definitive assessment of how effective the drug is in comparison with current gold standard treatment. A new Phase 3 trial evaluating levosimendan also known as ODM-109 an oral treatment by Orion for breathing problems in amyotrophic lateral sclerosis ALS has recruited its first patients.
Listing a study does not mean it has been evaluated by the US. 350 adults 90 trial sites 21 randomized Enrolled participants will receive ULTOMIRIS or placebo every eight weeks following an initial loading dose. A Phase 3 clinical trial is recruiting adults with amyotrophic lateral sclerosis ALS to evaluate the safety and effectiveness of NeuroNata-R lenzumestrocel a stem cell therapy approved to treat ALS in South Korea.
This study was designed in partnership with people living with ALS leading ALS specialists and global regulatory agencies. A Phase 3 trial of levosimendan an oral treatment by Orion for breathing problems in amyotrophic lateral sclerosis ALS is enrolling about 450 patients across North America Europe and Australia. A post-hoc analysis of subgroup outcomes and biomarkers in the phase 3 clinical trial EMPOWER of dexpramipexole in ALS.
Likely to be initiated in the coming months the trial to be called PHOENIX will be take place at 55 sites across the US. Ionis Pharmaceuticals is opening a Phase 3 safety and efficacy trial of ION363 jacifusen in amyotrophic lateral sclerosis ALS patients with confirmed mutations in the FUS gene a known cause of juvenile-onset disease. VITALITY-ALS Ventilatory Investigation of Tirasemtiv and Assessment of Longitudinal Indices after Treatment for a Year Cytokinetics is conducting a Phase 3 clinical trial for the drug Tirasemtiv.
Phase 3 clinical study. The ALS Association asked the FDA to skip a Phase 3 trial believed to be the only time the association has done that said Neil Thakur the ALS Associations chief mission officer. NASDAQBCLI is currently conducting a Phase 3 clinical trial of NurOwn in patients with amyotrophic lateral.
 Brainstorm Announces Topline Results From Nurown Phase 3 Als Study Nov 17 2020
Brainstorm Announces Topline Results From Nurown Phase 3 Als Study Nov 17 2020
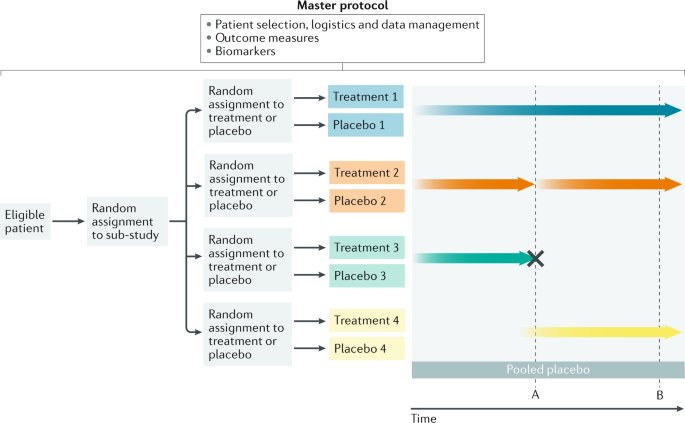 Improving Clinical Trial Outcomes In Amyotrophic Lateral Sclerosis Nature Reviews Neurology
Improving Clinical Trial Outcomes In Amyotrophic Lateral Sclerosis Nature Reviews Neurology
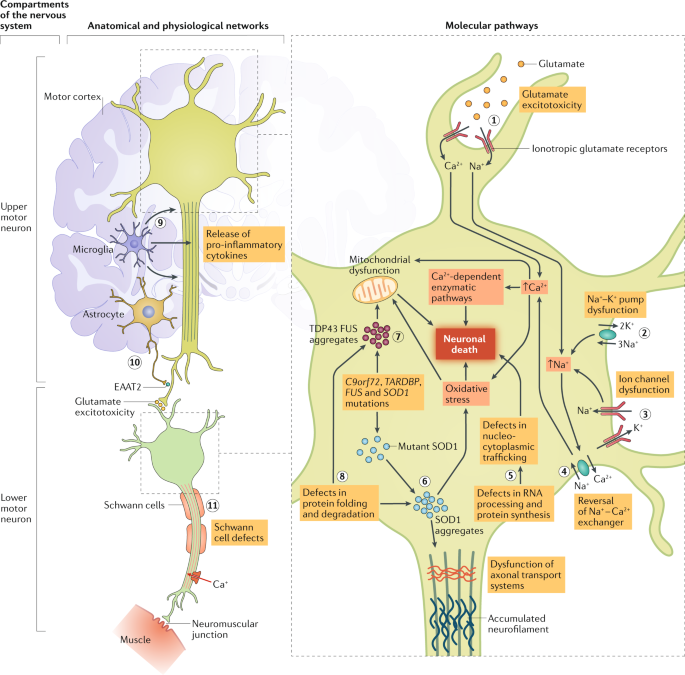 Improving Clinical Trial Outcomes In Amyotrophic Lateral Sclerosis Nature Reviews Neurology
Improving Clinical Trial Outcomes In Amyotrophic Lateral Sclerosis Nature Reviews Neurology
 Story 100 People With Als To Get Multiple Nurown Treatments In Phase 3 Clinical Trial Als Therapy Development Institute
Story 100 People With Als To Get Multiple Nurown Treatments In Phase 3 Clinical Trial Als Therapy Development Institute
 Add On Masitinib Slows Progression Of Als Phase 2 3 Trial Results Show
Add On Masitinib Slows Progression Of Als Phase 2 3 Trial Results Show
 Amylyx Pharma S Als Drug Needs A Phase 3 Study To Support Fda Submission Medcity News
Amylyx Pharma S Als Drug Needs A Phase 3 Study To Support Fda Submission Medcity News
Als Drug Slows Progression Petition For Fda Approval Als Texas
 Alexion Announces Planned Initiation Of Pivotal Phase 3 Study Of Ultomiris Ravulizumab In Als Business Wire
Alexion Announces Planned Initiation Of Pivotal Phase 3 Study Of Ultomiris Ravulizumab In Als Business Wire
 Ionis Opening Phase 3 Trial Of Ion363 Antisense Therapy For Fus Als
Ionis Opening Phase 3 Trial Of Ion363 Antisense Therapy For Fus Als
 Ionis Initiates Phase 3 Trial Of Novel Antisense Medicine To Treat Leading Cause Of Juvenile Onset Als Ionis Pharmaceuticals Inc
Ionis Initiates Phase 3 Trial Of Novel Antisense Medicine To Treat Leading Cause Of Juvenile Onset Als Ionis Pharmaceuticals Inc
 Als Phase 3 Trial To Test Alexion S Complement System Inhibitor Ultomiris
Als Phase 3 Trial To Test Alexion S Complement System Inhibitor Ultomiris
 Cytokinetics Plans Courage Als Phase 3 Als Trial To Test Reldesemtiv
Cytokinetics Plans Courage Als Phase 3 Als Trial To Test Reldesemtiv
 Als Canada Webinar Series Clinical Trials Als Society Of Canada
Als Canada Webinar Series Clinical Trials Als Society Of Canada
 Brainstorm S Stem Cell Therapy Flunks Phase 3 Als Trial Geneonline News
Brainstorm S Stem Cell Therapy Flunks Phase 3 Als Trial Geneonline News
Comments
Post a Comment