When Irvine California-based Axonics received FDA approval in September 2019 for its r-SNM system for fecal incontinence it not only became Medtronics sole competitor in the space but brought new capabilities as the first rechargeable version for patients while also being safe for patients to undergo full-body MRI scans. Patent and Trademark Offices Patent Trial and Appeal Board has decided to move forward in reviewing claims made by sacral neuromodulation specialist Axonics against Medtronic.
 Axonics Announce Positive Clinical Data From Its Artisan Sacral Neuromodulation Pivotal Study
Axonics Announce Positive Clinical Data From Its Artisan Sacral Neuromodulation Pivotal Study
Evidence from clinical trials shows that Axonics SNM system improves symptoms of overactive bladder and quality of life.

Axonics clinical trial. The RELAX-OAB Treatment of REfractory Overactive BLadder with the AXonics Sacral Neuromodulation System is a post-market clinical follow-up PMCF study designed to confirm the performance of the Axonics Sacral Neuromodulation SNM System as an aid in the treatment of the symptoms of overactive bladder OAB as well as capturing patient satisfaction and quality of life data. Axonics Modulation Technologies Inc. The EPG is a single-use non-rechargeable external device that.
The Axonics System is a miniaturized rechargeable SNM system designed to provide therapy for at least 15 years which is expected to significantly reduce revision surgeries as it will not require replacement as frequently as the non-rechargeable SNM system. Axonics Modulation Technologies Inc. The Axonics Trial Stimulator EPG is part of the Axonics SNM Trial System.
Omar Ford Jul 27 2020 Axonics Modulation Technologies has released topline clinical results from its ARTISAN-sacral neuromodulation SNM pivotal study. Axonics wins FDA nod for MR-safe neurostim device in trial June 18 2019By Sarah Faulkner Axonics Modulation TechnologiesNSDQAXNX said today that the FDA approved the use of full-body MRI for. The de novo model involved a quarterly progression between 3.
The safety and effectiveness of the Axonics Sacral Neuromodulation SNM System for urinary control was based on the results of a prospective multicenter clinical study designed to. The ARTISAN-SNM study is a 129-patient single-arm prospective multi-center unblinded pivotal clinical study to evaluate the safety and efficacy of the Axonics r-SNM System. Axonics Modulation Technologies Inc.
The Irvine CA-based companys Axonics r-SNM System was evaluated in a trial of 129 patients for the treatment of urinary and bowel dysfunction. AXNX a medical technology company that has developed and is commercializing novel implantable sacral neuromodulation SNM devices for the treatment. The battery is expected to last at least 6 years at.
Axonics Modulation Technologies Inc. Most clinical parameters in Noblett 2017 are derived from the 12month Insite trial Noblett 2016. Funding Axonics Modulation Technologies Inc.
Trial Stimulator or External Pulse Generator EPG. Clinical Trial Yes Registration Number NCT03327948 RCT No Subjects Human Ethics Committee IRB Helsinki Yes Informed Consent Yes 26022021 095659. And five centers in Western Europe.
The ARTISAN-SNM study is a 129-patient single-arm prospective multi-center unblinded pivotal clinical study to evaluate the safety and efficacy of the Axonics r-SNM System. The cost analysis suggests that using Axonics SNM system may lead to cost savings but this depends on the length of time the battery lasts. The study was conducted in 14 centers in the US.
36 The de novo cost analysis used a Markov model adapted from a previously published model Noblett 2017 to compare the rechargeable Axonics SNM system with the non-rechargeable system in people with overactive bladder. AXNX a medical technology company that has developed and is commercializing novel implantable sacral neuromodulation SNM devices for the treatment of urinary and bowel dysfunction has reported full 2-year clinical results from its ARTISAN-SNM pivotal study. AXNX a medical technology company that has developed and is commercializing novel implantable sacral neuromodulation SNM devices for the treatment.
Irvine California-based Axonics gained an FDA approval one year ago to become Medtronics first challenger in the market for neurostimulators to treat. AXNX a medical technology company that has developed and is commercializing novel implantable sacral neuromodulation SNM devices for the treatment.
Axonics Sacral Neuromodulation Overactive Bladder Urinary Retention
 Axonics Reports 2 Year Clinical Results From Artisan Snm Pivotal Study Business Wire
Axonics Reports 2 Year Clinical Results From Artisan Snm Pivotal Study Business Wire
 Axonics System For Sacral Neuromodulation Overview
Axonics System For Sacral Neuromodulation Overview
 Axonics Announces Two Year Results From Its Artisan Snm Study
Axonics Announces Two Year Results From Its Artisan Snm Study
 Axonics System For Sacral Neuromodulation Overview
Axonics System For Sacral Neuromodulation Overview
 Fda Approves Axonics Rechargeable Sacral Neuromodulation Device
Fda Approves Axonics Rechargeable Sacral Neuromodulation Device
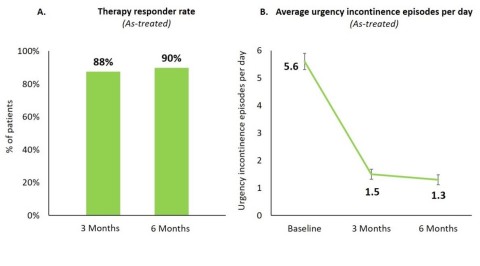 Axonics Announces Positive Top Line Clinical Data From Its Artisan Snm Pivotal Study Biospace
Axonics Announces Positive Top Line Clinical Data From Its Artisan Snm Pivotal Study Biospace
 First Patients Implanted With Axonics Sacral Neuromodulation System In Fda Pivotal Study Medical Product Outsourcing
First Patients Implanted With Axonics Sacral Neuromodulation System In Fda Pivotal Study Medical Product Outsourcing

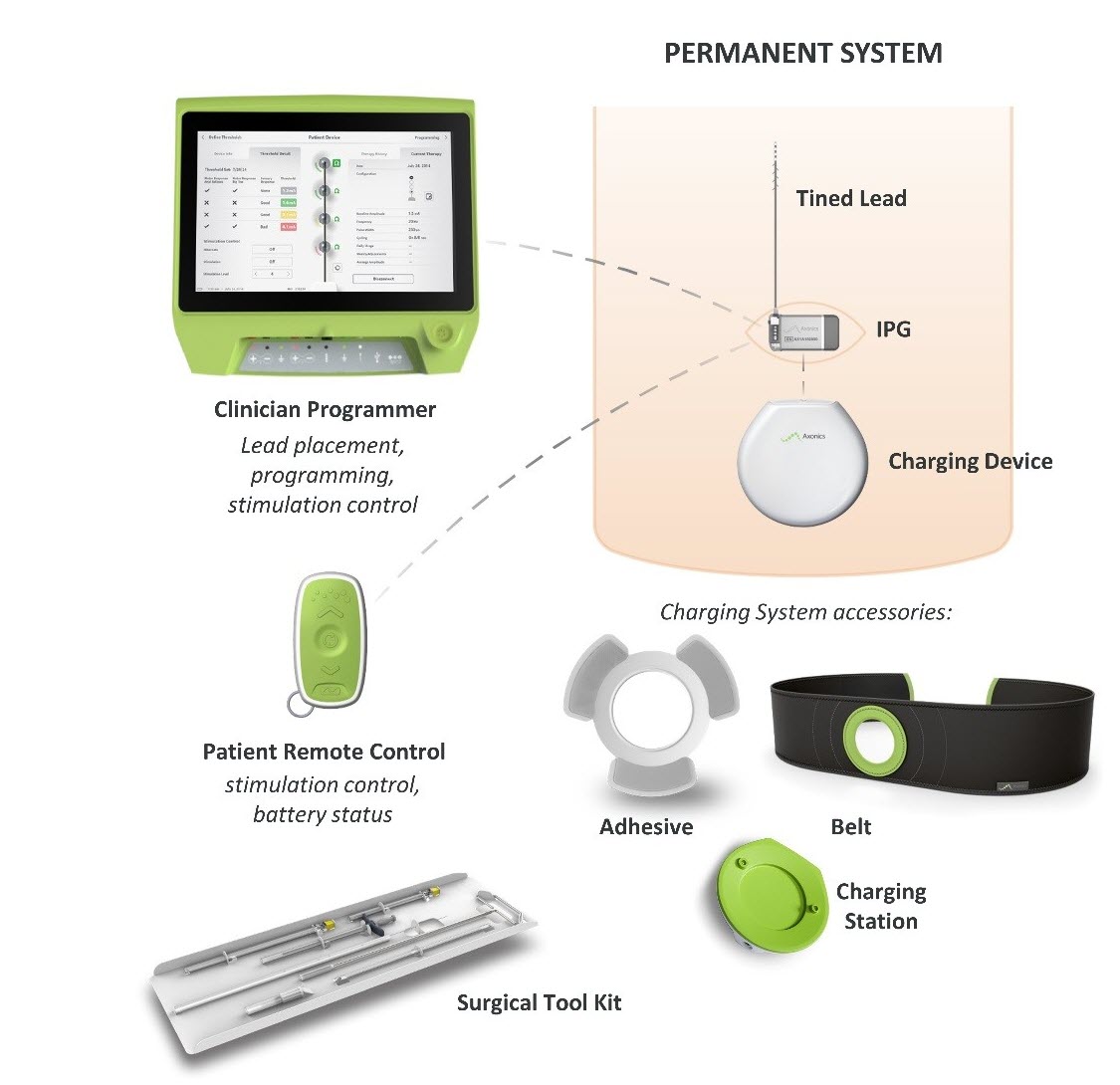 Axonics Sacral Neuromodulation Snm System For Urinary Control P180046 Fda
Axonics Sacral Neuromodulation Snm System For Urinary Control P180046 Fda
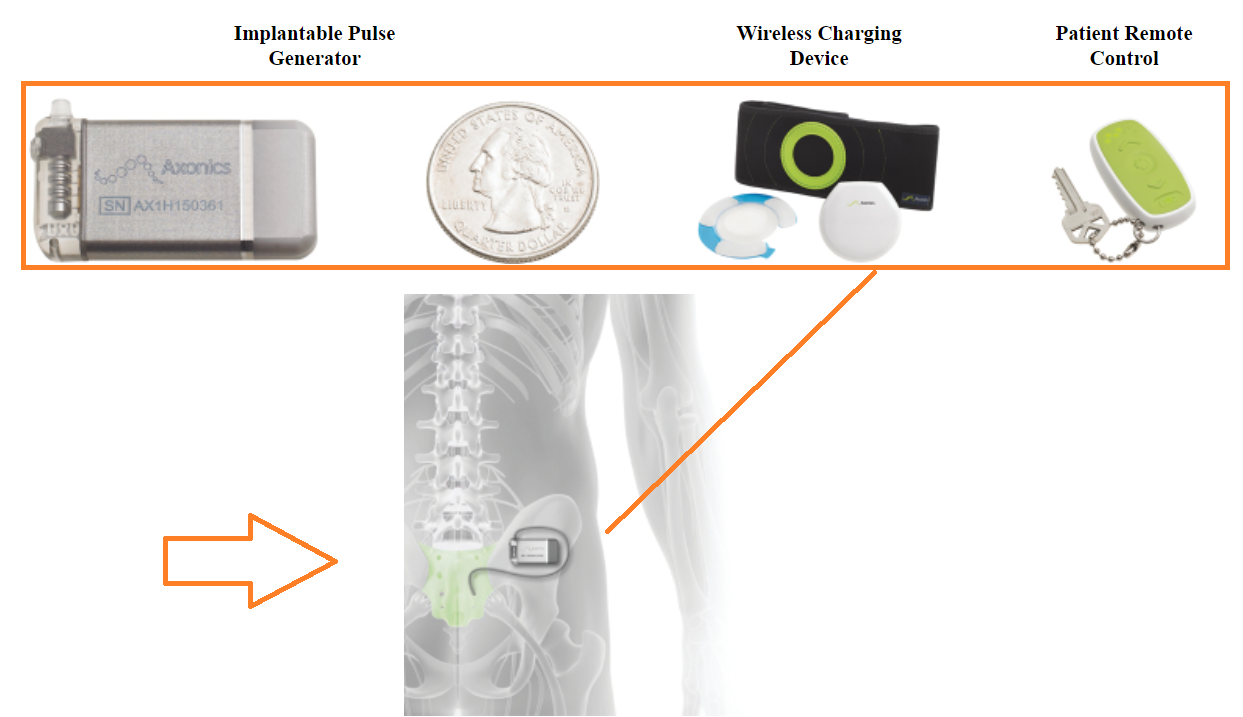 Axonics Modulation Ipo Data To Be Released In 2019 Nasdaq Axnx Seeking Alpha
Axonics Modulation Ipo Data To Be Released In 2019 Nasdaq Axnx Seeking Alpha
 Axonics System For Sacral Neuromodulation Overview
Axonics System For Sacral Neuromodulation Overview
 Axonics Modulation Ipo Data To Be Released In 2019 Nasdaq Axnx Seeking Alpha
Axonics Modulation Ipo Data To Be Released In 2019 Nasdaq Axnx Seeking Alpha

Comments
Post a Comment